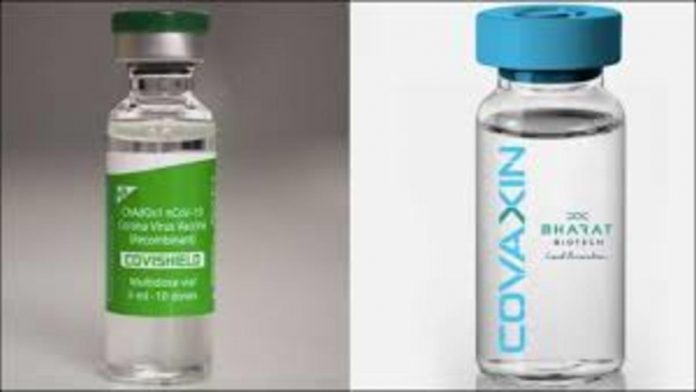
Covid vaccines Covaxin and Covishield have been granted regular market permission by an expert panel of India’s central drug authority. This market approval means that the vaccines can be used without reservations or conditions.
Taking to twitter, the Central Drugs Standard Control Organization (CDSCO) confirmed the news saying, “SEC has recommended for upgrade of Covishield and Covaxin status from restricted use in emergency situations to grant of new drug permission with conditions in the adult population, DCGI will evaluate the recommendations and give its decision.”
“The Subject Expert Committee (SEC) on Covid-19 of the Central Drugs Standard Control Organisation (CDSCO) which reviewed SII and Bharat Biotech’s application for the second time on Wednesday has recommended granting regular market approval to Covishield and Covaxin subject to certain conditions,” an official source said.
When asked about how these vaccine will be marketed, then a source told that it will be available at all the hospitals and clinics registered with CoWin and at the time of administration the hospitals and clinics will have to update it on CoWin.
The Bharat Biotech and Serum Institute of India (SII) had requested the DCGI for regular market authorization.